Minireview Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Electrochemical Treatment of Tumours: Brief Considerations
*Corresponding author: Cesar Augusto Correia de Sequeira, Department of Chemical and Biological Engineering, Instituto Superior Técnico, University of Lisbon, Av Rovisco Pais, 1049-001 Lisboa, Portugal.
Received: September 15, 2022; Published: September 22, 2022
DOI: 10.34297/AJBSR.2022.17.002317
Abstract
The tumour is treated by electrochemical treatment (ECHT) or electrolytic ablation neoplastic tissue with a continuous direct current through two or more electrodes located inner side or nearby the tumour. The ECHT has been taken considerable attention as being one of the numerous methods for malignancies local treatment. The therapy benefit is the negligibly invasive procedure and few serious side effects. Despite treating people around the world in recent years, ECHT has not been globally accepted yet. This brief article deals with the basics of ECHT, namely the electrolytic method for tumours treatment and the destructive mechanisms, whose uncertainties hinder the development of an optimized and dependable procedure for serving as a complement to the oncologic treatments utilized in the Western world.
Keywords: Apoptosis; Necrosis; Cancer; Direct Current; Electrotherapy; Tumour Electrolysis; Anti-Tumoural Treatment; Electrolytic Ablation
Introduction
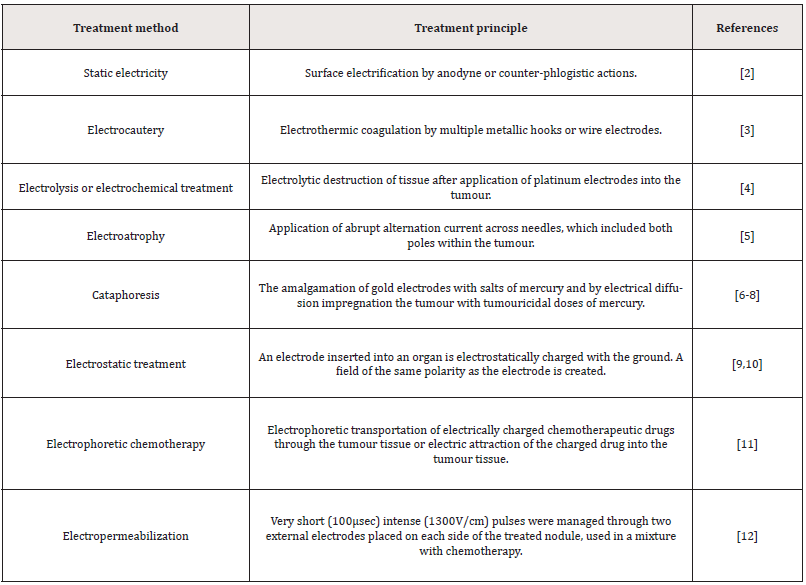
In electrochemical treatment (ECHT) or electrolytic ablation, a direct electric current streams through the tumour cellular and interstitial compartments, the latter including mostly a complex conglomerate of collagen, glycoproteins, proteoglycans and hyaluronic acid. Tissue destruction has been described via this procedure in an extensive range of solid tumours, with greater effectiveness detected in skin cancer, oral cavity and thyroid malignancies [1]. In tissue destruction, some contributory components look to be involved. But these respective characteristics in creating the anti-tumours effects have not been fully comprehended. The electricity power means fascination and its usage in oncology spans from the late 18th century [2,3]. A summary of the landmarks in the utilisation of different electrical usages has been illustrated in (Table 1). The ECHT usage Information could be discovered as far back in history as the mid- 19th century [4-12]. While it is tough to critical evaluation these reports a few attempts have been made to express the ECHT usage [1,13-15].
The electrochemical treatment in contemporary history began in 1959 when Humphrey and Seal described encouraging consequences with sarcoma tumours in mice [16]. After this several experiments were led in which animal tumour models and in vitro tests would serve as the base for the ECHT introduction in the clinical setting. It is suitable at this point to attend that, although recognized since the 19th century middle, Bjorn Nordenstrom, from Sweden, is considered to be a forerunner in tumour therapy with electric current and mixture treatments in patients [9,10]. In the late seventies, primary lung cancers were started to be treated via applying current between two platinum wire electrodes by Nordenstrom and, in his book of 1983 [9], he reported results from the treatment of 26 lung tumours in 20 patients. Relapse was achieved in 12 out of 26 tumours and regrowth symptoms were not determined after a 2–5-year follow-up period.
Following Nordenstrom’s works, Xin Yu-Ling and his group in China extended ECHT to the whole country (more than 1500 patients have been treated in the period 1990-2005) [17-19]. Initially, due to the low quality, the studies were not taken seriously in the Western world. A few years later, the procedure has been changed and many articles have been established by groups in Europe, the USA and Australia that utilize a mixed variety of the methods reported by the Chinese [20-23]. Moreover, in 1991, the effects of ECHT on tumours in mice were investigated by Miklavčič et al in Slovenia in a series of articles [24-27]. Especially, in 1992, Serša et al investigated the antitumour effectiveness of electrochemical treatment in combination with anticancer drugs like bleomycin [28]. Fifteen years later, Von Euler et al reported ECHT treatment effectiveness on cell proliferation and apoptosis in rat mammary cancer [29]. Nowadays, several groups are working in Australia, Cuba, Japan, Slovenia, Sweden and USA [30-40].
Some of the ECHT benefits are its simplicity, effectiveness, inexpensive and few side effects. This treatment is especially shown for superficial, non-operable, or chemotherapy-resistant tumours. It has also been recommended that the ECHT would potentiate the antineoplastic effects of radio and chemotherapy and diminish their side effect [10,41]. The induced transmembrane potential in a cell subjected to an electric field leads to an increment in membrane permeability, so permitting specific molecules to be transferred into the cell [42]. This procedure is regularly named electroporation or electropermeabilization or irreversible electroporation (IRE) and has been broadly utilized in molecular biology and associated fields [37,38]. In comparison to thermodependent modalities for tissue ablation (e.g. radiofrequency ablation, microwave ablation, laser interstitial therapy, highintensity focused ultrasound and cryoablation), IRE which is a commonly utilized thermal-independent modality, destroys cancer cells by interrupting membrane integrity [43]. IRE exerts high electric potential microsecond pulses (up to 3000V) between two or more electrodes [38].
Whereas the tendency for heat to be produced scales with the voltage applied amplitude, IRE does not mechanistically depend on hyperthermia to lead to cell death. It is a widely held view that this cell dead instead moves from the induced transmembrane potential which irretrievably disrupts the lipid bilayer integrity; particularly, in order for cell death to happen, a potential of 1-2V across a cell membrane is needed [44,45]. This method’s specific advantage is that the extracellular matrix keeps mostly intact. The IRE primary defects are secondary side effects related to the high magnitude of the applied voltages. The transported pulses voltages have the potential to induce cardiac arrhythmias and muscle contractions, which make necessary the usage of general anaesthesia [38]. Moreover, precise electrode alignment is needed to ensure sufficient charge deposition and to alleviate thermal injury to nontarget tissues [46].
Despite relating electrochemotherapy (ECT) and gene electrotransfer procedures to IRE, they are distinguished via their usage of either fewer electrical pulses or lower voltage magnitudes, respectively. These modalities induce impermanent and cell membranes sublethal permeabilization that simplifies the cargo delivery to cells. Electrochemotherapy is utilized in cancer treatment to increase the uptake of chemotherapeutic agents. Gene electrotransfer is similar to electrochemotherapy, however, instead simplifies the transfer of a gene or genes to cells to enable the downstream production of a therapeutic protein [47]. Whereas these methods illustrate similar restrictions to those observed with IRE, their usefulness matters from their selectivity and wellcharacterized mechanisms of action. ECHT, also recognized as electrochemical treatment causes necrosis and apoptosis via the application of a direct current between multiple electrodes at relatively low electric potentials in comparison with IRE, sometimes lower than 50 V. Therefore, this method suggests unique benefits in comparison with other ablation procedures [31].
Particularly, electrolytic ablation may permit the development of exactly defined, shapeable ablation zones, that are uninfluenced via heat sink effects and could be checked and regulated in realtime by magnetic resonance imaging (MRI) [1,29,48]. Electrolytic ablation has been used for the therapy of various kinds of human malignancies; although, its progress has been restricted by uncertainty considering the underlying mechanism of induced cell death [31,49,50]. Whereas it has been well proved that electrolytic ablation induces electrolysis, as argued in the next section, a diversity of potential mechanisms have been recognized that may underlie the detected cell death. These comprise electric charge deposition, the making of a cellular transmembrane potential, toxic materials production, water extraction by electroosmosis, and alteration of microenvironmental pH [51-54]. Cell death mechanistic comprehending induced by electrolytic ablation has been needed to enable its additional progress for extensive clinical application.
Moreover, comprehending cellular alteration induced via electrolytic ablation will increase parameter choice for associated electrochemical treatments, comprising IRE, electrochemotherapy, and gene electrotransfer, that might include synergistic effects when compounded with electrolytic ablation [40,55]. Following this introductory review we briefly describe the mechanism by which the ECHT causes cell death, then we report the main destructive mechanisms, and finally, for a better understanding the fundamental electrolytic mechanisms involved, we describe the combined methodology proposed for studying ECHT in tumours, which are a powerful tool for viewing this complex problem while assuring that experimental and /or numerical artefacts are more easily detected.
ECHT Principle
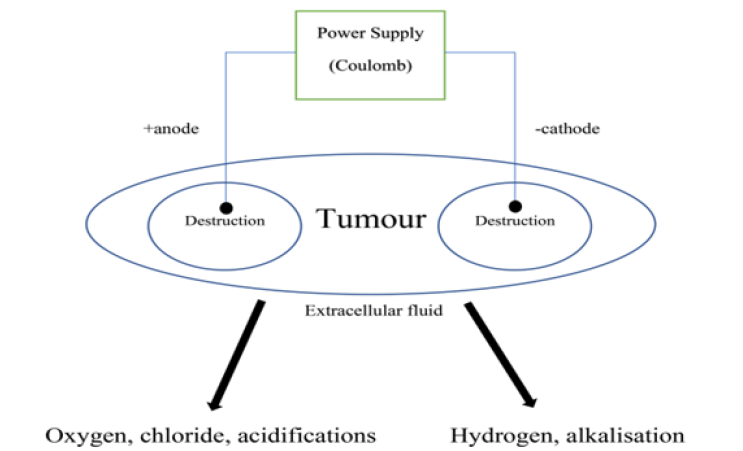
When two or more electrodes have been injected inside of the tissue a series of electrochemical reactions have been occurring. When a practically insolvable electrode substance like platinum has been utilized, the main electrode reactions are the water splitting with materials oxidation and reduction solved in the tissue. At the anode the oxygen evolution, along with chlorine acidification and formation, happen:
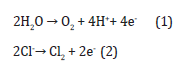
Moreover, chlorine may react with water causing further acidification:
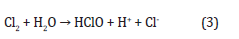
Regarding experimental results along with theoretical assessments, the hydrogen ions spreading in the tissue surrounding the anode are significantly larger than the chlorine spreading [56]. At the cathode, water is broken down into hydrogen and hydroxyl ions:
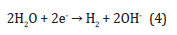
Considering the acidic and basic heamatin formation, the tissue around the anode and cathode converts to dark brown [57]. The acidic heamatin comprises methaemoglobin whereas the dark area around the cathodic lesion includes haemochromogens [58, 59]. Due to the spreading of The chlorine, a grey-coloured zone near the anode is created, which is noticeably smaller than the heamatin area [58].
The delivered charge (dose) has been usually described in Coulomb (C). Coulomb is the electrical charge unit equal to the charge amount delivered by a current of 1 ampere in 1 second (As). Diverse plans have been applied for therapies; some investigators have utilized constant voltage and a variable current, whereas others have remained the current constant to let the voltage vary instead. For both methods, the Coulomb dose could be detected. EChT treatment and electrolysis schematic have been depicted in (Figure 1).
The Destructive Mechanisms
Many suggestions have been made on which should be the main cause of tissue destruction after electrochemical treatment. According to some scientists’ reports, the electric field has a significant impact on cell death or tumour tissue remodelling [60,61]. The electric field leads to interstitial water flux, and electro-osmosis, from the anode to the cathode, since the water molecules operate as a dipole. Therefore, the tissue around the anode dehydrates whereas oedema has been obtained around the cathode [9,61]. Charged materials, solved or suspended in tissue, transferred in the electric field and ions accumulation and charged tissue constituents have been acquired at specified and diverse zones in the electric field. The electric field affects the ion exchange across the cell membranes. Thus, the membrane potential has been changed and therewith the circumstances e.g. for many vital enzyme-regulated reactions [9,60].
Chlorine (CI) has been considered as being the most toxic agent by other scientists [59,62]. With little hesitation though, the extreme pH gradient that happens at ECHT has been explained by most articles. At the anode, as low pH as 1 has been evaluated [9,17]. Whereas at the cathode the prominent alkalisation produces pH levels as high as 13 [9,63]. Due to extreme pH amounts, the tissue proteins convert denaturated and the cell structure collapses and the cell finally dies [9,57,63]. The pH changes during ECHT have also been predicted by many theoretical computations. The hydrogen ions and molecular chlorine spreading around spherical and planar platinum anodes that have been investigated by Berendson et al. [56,64]. The formation and spreading of potential toxic species around both the anode and cathode, rather with a spherical electrode configuration have been studied by Nilsson et al. [54,65,66].
The above are true in an environment where the therapy electrodes are inert. If the electrode has been made from a soluble substance like copper (Cu), rhodium (Rh) or brass (Zn-Cu alloy) the electrolysis leads to metal ions solving in the tissue [67]. Therefore, the metal ions could include toxic capacity by themselves. Miklavčič, Serša et al, have illustrated both the pH change importance in tissue during ECHT along with the metal ions formation during electrolysis when utilizing other materials than platinum (Pt) [26]. In another research, they also revealed that the intralesional temperature change is marginal and hence, most likely, does not influence cell survival [27].
To sum up, the results described above demonstrate that ECHT of cancer results from the introduction of toxic acid and base that modulates the tumour microenvironment primarily involving a pH-dependent mechanism that mitigates the limitations of leading ablation modalities. Studies in progress demonstrate that cancer cells placed in an environment at a pH below 4.8 or above 10.6 undergo cell death. These unique insights will be essential for leveraging cancer cell susceptibility to altered microenvironments as well as furthering the development of electrolytic ablation for clinical application.
In Vivo, In Vitro and In Silico Modeling
Animal models have an important role in the investigation of human cancer. One of the major causes is that both have almost the same genes complement and signal pathways (tumours in mice and humans mutate in the same genes class which is an index of similar mechanisms leading tumour growth). Actually, the article reveals that for better preclinical modelling, mice are appropriate [68]. In the human tumour invitro EChT modeling has been utilized a gel of collagen I expose to an electric field where it has been supposed that the gel includes physicochemical and hydrodynamic properties near to those found in the solid tumour interstitium. Collagen usage is according to the fact that it comprises more than 70% of the tumour interstitium. Furthermore, the previous document indicates that collagen type I gels might be a good initial simplified model of the transport of species in the tumour extracellular matrix [69,70]. a gel of agar has been also used because it is more appropriate for optical studies and more chemically resistant.
In silico cancer modeling is an effective tool that could supply more vision into the mechanisms that control tumour evolution and growth. It points both mathematical modeling and numerical simulation. Generally, it comprises in a system of reaction-transport differential equations in a fixed or moving domain (Stefan-like problem) explaining physicochemical conservation laws whose solution has been achieved by numerical techniques. Presently, the Preziosi book could be accessed as an effective area of research and a review [71]. Jain et al did revolutionary work in the subject [72-75]. Nilsson et al in a series of articles developed EChT in silico modeling [53,54,65,66]. They described ion transport in an area close to one of the electrodes (cathode or anode) via a quasi-onedimensional model utilizing the Nernst-Planck equations for ion transport under the electroneutrality hypothesis. Considering their initial model, the tissue matrix was approximated to a saline (NaCl) solution with a specified buffer volume and organic content.
Regarding consecutive refinements, the model was extended to comprise the bicarbonate buffer effects on anodic hydrogen ions and the transfer and reaction of chlorine and chlorinated species. The consequences were compared with experimental information from in vivo rat normal tissue giving a good explanation of the n’ profile near the anode after ECHT. In their final model [54] ion transfer close to the cathode in which they reveal their simulated pH profiles to be strongly associated with the size of experimentally calculated lesions has been investigated by them, so approving that the spreading of hydroxyl ions detected the lesion size around the cathode. They also recommended that the model could be utilized for anticipating the size of the lesion produced by ECHT..
References
- Nilsson E, Von Euler H, Berendson J, Thorne A, Wersall P, et al. (2000) Electrochemical treatment of tumours. Bioelectrochemistry 51(1): 1-11.
- Carvalho T (1777) A Complete Treatise on Electricity in Theory and Practice, Dilly, London, UK.
- Duchenne G (1872) de L’Electrisation Localisée, Baillière, Paris, France.
- Crussel G (1847) Die Electrilytishen Heilanstalt in Moscow, Med Zeitung. Russlands 4: 2041.
- Inglis Parsons J (1893) The Healing of Rodent Cancer by Electricity, Bala, London, UK.
- Massey GB (1898) Conservative Gynecology and Electro-Therapeutics, F.A. Davis Co., Philadelphia, USA.
- Massey GB (1914) Ionization treatment of cancer. End-result of twenty years’ work. A summary of 300 cases, Amer J Surgery 28: 329.
- Massey GB (1924) Practical Electrotherapeutics and Diathermy, MacMillan, New York, USA.
- Nordenström BE (1983) Biologically Closed Electrical Circuits: Clinical, Experimental and Theoretical Evidence for an Additional Circulatory System, Nordic Medical Publications, Stockholm, Sweden.
- Nordenström BE (1989) Electrochemical treatment of cancer I: Variable response to amodic and cathodic fields. Am J Clin Oncol 12(6): 530-536.
- Nordenström BE, Eksborg S, Beving H (1990) Electrochemical treatment of cancer II: Effect of electrophoretic influence on Adriamycin. Am J Clin Oncol 13(1): 75-88.
- Mir L, Belehradek M, Domenge C, Orlowski S, Poddevin B, et al. (1991) Electrochemotherapy, a novel antitumour treatment: first clinical trial. C R Acad Sci III 313(13): 613-618.
- Nordenström BE (1994) Survey of mechanisms in electrochemical treatment (ECHT) of cancer, European J Cancer (Suppl 574): 93-109.
- Schechter DC (1979) Flashbacks: Containment of tumour through electricity. Pacing Clin Electrophysiol 2(1): 100-114.
- Watson (1991) The treatment of tumours with direct electric current. Medical Science Research 19: 103-105.
- Humphrey CE and Seal EH (1959) Biophysical approach toward tumour regression in mice. Science 130(3372): 388-390.
- Xin YL (1994) Organization and spread of electrochemical therapy (ECT) in China. Eur J Surg Suppl 1994(574): 25-29.
- Xin YL (1998) The Clinical advance in application of ECHT within the past ten years, Preprints 2nd Symp on Electrochemical Treatment of Cancer 27-30 Beijing, China 81-92.
- Matsushima Y, Takahashi E, Magiwara K, Konaka C, Miura H, et al. (1994) Clinical and experimental studies of antitumoural effects of electrochemical therapy (ECT) alone or in combination with chemotherapy. European J Surgery 574: 59-67.
- Robertson GS, Wemyss Holden SA, Dennison AR, Hall PM, Baxter P, et al. (1998) Experimental study of electrolysis induced hepatic necrosis. Br J Surg 85(9): 1212-1216.
- Turler A, Schaefer H, Schaefer N, Maintz D, Wagner M, et al. (2000) Local treatment of hepatic metastases with low-level direct electric current: experimental results. Scandinavian J Gastroenterology 35(3): 322-328.
- WemyssHolden SA, Hall PM, Robertson GS, Dennison AR, Vanderzon PS, et al. (2000) The safety of electrolytically induced hepatic necrosis in a pig model. Aust N Z J Surg 70(8): 607-612.
- Wemyss Holden SA, Robertson GS, Dennisson AR, Vanderzon PS, Hall PM, et al. (2000) A new treatment for unresectable liver tumours: long-term studies of electrolytic lesion in the pig liver. Clin Sci (Lond) 98(5): 561-567.
- Miklavcic D, Sersa G, Novakovic S, Rebersek S (1990) Tumour Bioelectric potential and its potential explotation for tumour growth retardation. J Bioelectricity 9(2): 133-149.
- Sersa G, Miklavcic D (1993) The fearsibility of low-level direct current electrotherapy for regional cancer treatment. Regional Cancer Treatment 6: 31-35.
- Miklavcic D, Fajgelj A, Sersa G (1994) Tumour treatment by direct electric current: electrode material deposition. Bioelectrochemiestry and Bioenergetics 35(1-2): 93-97.
- Miklavcic D, Sersa G, Kryzanowski M, Novakovic S, Bobanovic F (1993) Tumour treatment by direct electric current: tumour temperature and ph, electrode material and confirmation. Bioelectrochemistry and Bioenergetics 30: 209-220.
- Sersa G, Novakovic S, Miklavcic D (1993) Potentiation of bleomycin antitumor effectiveness by electrotherapy. Cancer Lett 69(2): 81-84.
- Von Euler H, Strahle K, Thorne A Younging G (2004) Cell prolifration and apoptosis in rat mammary cancer after electrochemical treatment (ECHT). Bioelectrochemistry 62(1): 57-65.
- Sequeira CAC, Cardoso DSP (2014) Electrotherapy, a recent mode for anticancer treatment. Science and Technology of Materials 26(2): 126-130.
- Ciria HMC, González MM, Zamora LO, Cabrales LEB, González GVS, et al. (2013) Antitumour effects of electrochemical treatment. Chin J Cancer Res 25(2): 223-234.
- Olaiz N, Maglietti F, Suarez C, Molina F, Miklavcic D, et al. (2010) Electrochemical treatment of tumours using a one-probe two-electrode device. Electrochimica Acta 55(20): 6010-6014.
- Tiong LU, Finnie JW, Field JB, Maddern GJ (2012) Bimodal electric tissue ablation (BETA)- effect of reversing the polarity of the direct current on the size of ablation. J Surg Res 174(2): 305-311.
- Turjanski P, Olaiz N, Maglietti F, Michinski S, Suarez C, et al. (2011) The role of pH fronts in reversible electroporation, PLoS One 6(4): e17303.
- Cabuy E (2012) Electrochemical therapy of cancer treatment, Reliable Cancer Therapies. Energy-based therapies 6: 1-20.
- Qi G, Wang B, Song X, Li H, Jin Y (2020) A green, efficient and precise hydrogen therapy of cancer based on in vivo electrochemistry. National Science Review 7(3): 660-670.
- Chu KF, Dupuy DE (2014) Thermal ablation of tumours: biological mechanism and advances in therapy. Nat Rev Cancer 14(3): 199-208.
- Knavel EM, Brace CL (2013) Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol 16(4): 192-200.
- Vroomen LGPE, Petre EN, Cornelis FH, Solomon SB, Srimathveeravalli G (2017) Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone; what are the differences? Diagn Interv Imaging 98(9): 609-617.
- Phillips M, Krishnan H, Raju N, Rubinsky B (2016) Tissue ablation by a synergistic combination of electroporation and electrolysis delivered by a single pulse. Ann Biomed Eng 44(10): 3144-3154.
- Ciria HMC, Quevedo M, Cabrales R, Bruzon R, Salas M, et al. (2004) Antitumor effectiveness of different amounts of electrical charge in Ehrlich and fibrosarcoma sa-37 tumors. BMC Cancer 4: 87.
- Newmann E, Rosenbec K (1972) Permeability changes induced by electric impulses in vesicular membranes. J Membrane Biology 10(3): 279-290.
- Miller L, Leon J, Rubinsky B (2005) Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat 4(6): 699-705.
- Davalos RV, Mir LM, Rubinsky B (2005) Tissue ablation with irreversible electroporation. Ann Biomed Eng 33(2): 223-231.
- Kurata K, Matsushita M, Yoshii T, Fukunaga T, Takamatsu H (2012) Effect of irreversible electroporation on three-dimensional cell culture model. Conf Proc IEEE Eng Med Biol Soc 2012: 179-182.
- Adeyanju O, Al Angari H, Sahakian A (2012) The optimization of needle electrode number and placement for irreversible electroporation of hepatocellular carcinoma. Radiol Oncol 46(2): 126-135.
- Mir LM (2006) Bases and rationale of the electrochemotherapy. Eur J Cancer 4(11): 38-44.
- Wemyss Holden SA, Dennison AR, Finch GJ, Hall PM, Maddern GJ (2002) Electrolytic ablation as an adjunct to liver reaction: experimental studies of predictability and safety. Br J Surg 89(5): 579-585.
- Wang H (1994) Electrochemical therapy of 74 cases of liver cancer. Eur J Surg 574: 55-57.
- Wu G, Zhou X, Huang M (2001) Electrochemical therapy and implanted ports treatment for unresectable carcinoma of body and tail of pancreas. Chin J Surg 39(8): 596-598.
- Kotnik T, Miklavcic D (2006) Theoretical evaluation of voltage inducement on internal membranes of biological cells exposed to electric fields. Biophys J 90: 480-491.
- Vijh A (2006) Phenomenology and mechanisms of electrochemical treatment (ECT) of tumours. Mod Asp Electrochem 39: 231-274.
- Nilsson E, Berensdon J, Fontes E (2000) Impact of chlorine and acidification in the electrochemical treatment of tumours. J Appl Electrochem 30: 1321-1333.
- Nilsson E, Fontes E (2001) Mathematical modelling of physicochemical reaction and transport processes occurring around a platinum cathode during the electrochemical treatment of tumours. Bioelectrochemistry 53(2): 213-224.
- Marino M, Olaiz N, Signori E, Maglietti F, Suárez C, et al. (2017) pH fronts and tissue natural buffer interaction in gene electrotransfer protocols. Electrochim Acta 255: 463-471.
- Berendson J, Olsson JM (1998) Bioelectrochemical aspects of the treatment of tissue with direct current. Electro and Magnetobiology 17(1): 1-16.
- Lemberg R, Legge M (1949) Hematin Compounds and Bile Pigments. Interscience Publ Inc, New York, USA.
- Samuelsson L, Jonsson L (1981) Electrolytic destruction of tissue in the normal lung of the pig. Acta Radiol Diagn (Stockh) 22(1): 9-14.
- Samuelsson L, Olin T, Berg NO (1980) Electrolytic destruction of lung tissue in the rabbit. Acta Radiologica 21(4): 447-454.
- Vodovnik L, Miklavcic D, Sersa G (1992) Modified cell proliferation due to electrical currents. Medical and Biological Engineering 30(4): 21-28.
- Vijh AK (1999) Electrochemical treatment of tumors (ECT), Electroosmotic dewatering (EOD) as the primary mechanism. Drying Technology 17(3): 585-596.
- Samuelsson L, Jonsson L (1980) Electrolyte destruction of lung tissue. Electrochemical aspects. Acta Radiol Diagn (Stockh) 21(6): 711-714.
- Li K, Xin Y, Gu Y, Xu B, Fan D, et al. (1997) Effects of direct current on dog liver: possible mechanisms for tumor electrochemical treatment, Bioelectromagnetics 18(1): 2-7.
- Berendron J, Simonsson D (1994) Electrochemical aspects of the treatment of tissue with direct current , European J Surgery 574: 111-115.
- Nilsson E, Fontes E, Berendson J (1998) Electrochemical treatment of tumours: A simplified mathematical model, Part I. Bioelectrochemistry and Bioenergetics 47: 11-18.
- Nilsson E, Fontes E, Berendson J (1999) Electrochemical treatment of tumours: a simplified mathematical model. J Electroanal Chem 460(1-2): 88-99.
- Samuelsson L, Lamm I L, Jonsson L, Linden C J, Ewers SB (1991) Electrolysis with different electrode materials and combined with irradiation for treatment of experimental rat tumors. Acta Radiol 32(2): 178-181.
- Knowles M (2005) Introduction to the Cellular and Molecular Biology of Cancer, 4th Edn Oxford University Press, Oxford, UK.
- Netti P, Berk D, Swartz M, Grodzinsky A, Jain R (2000) Role of extracellular mattrix assembly in interstitial transport in solid tumors. Cancer Res 60(9): 2497-2503.
- Ramanujan S, Pluen A, Mckee T, Brown E, Boucher Y, et al. (2002) Diffusion and convention in collagen gels: implication for transport in the tumour interstitium. Biophysics J 83(3): 1650-1660.
- Preziosi L (2003) Cancer Modelling and Simulations. Chapman and Hall/ CRC, London, UK.
- Baxter LT, Jain R K (1989) Transport of fluid and macromolecules in tumours. I. Role of interstitial pressure and convention. Microvascular Research 37(1): 77-104.
- Baxter LT, Jain R K (1989) Transport of fluid and macromolecules in tumours. II. Role of heterogeneous perfusion and lymphatics. Microvascular Res 40(2): 246-263.
- Baxter LT, Jain R K (1991) Transport of fluid and macromolecules in tumours. III. Role of binding and metabolism, Microvascular Research 41(1): 5-23.
- Baxter LT, Jain R K (1991) Transport of fluid and macromolecules in tumours. IV. A microscopic model of the perivascular distribution, Microvasc Res 41(2): 252-272.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.